Ammonia G Oxygen G Nitrogen Monoxide G Water G
Temperature - Carbon Monoxide Gas - CO - specific heat of at temperatures ranging 175 - 6000 K. B Nitrogen and oxygen from the petrol react together in the car exhaust.
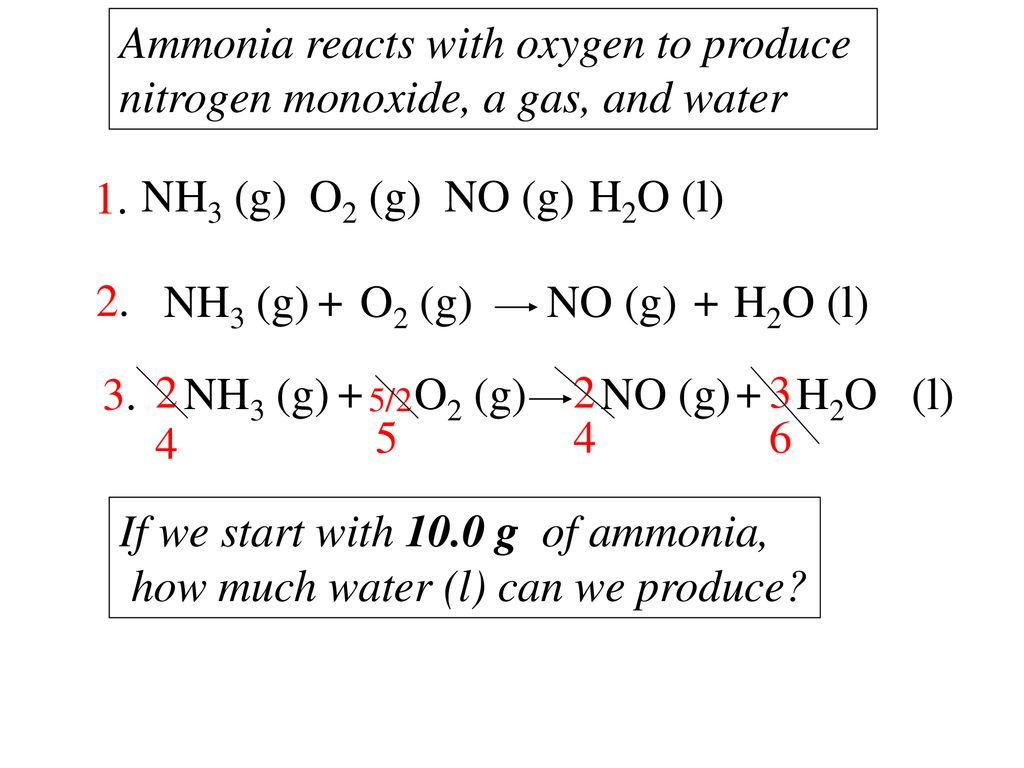
Balanced Chemical Reaction Ppt Download
1 H-atom abstraction and 2 3O2 addition reaction.
. CaCO₃s CaOs CO₂g In some compounds the energy needed for decomposition is so small that it can be supplied by a minor shock such as a physical impact. Online Ammonia Gas Density Calculator. Biologically it is a common nitrogenous waste particularly among aquatic organisms and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a.
When a chemical reaction occurs the. Density unit converter. Carbon Monoxide - Specific Heat vs.
28 The exhaust gases from cars contain oxides of nitrogen. In nature nitrogen-containing substances undergo complex metabolism and chemical changes to realize the cycle of nitrogen. A comparison of the pathways of these initial reactions and the search for a less energy-demanding pathway.
It is a common element in the universe estimated at seventh in total abundance in the Milky Way and the Solar SystemAt standard temperature and pressure two atoms of the element bind to. Carbon Monoxide and Health Effects - Exposure to Carbon Monoxide - CO and health effects. C Nitrogen reacts with oxygen.
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3A stable binary hydride and the simplest pnictogen hydride ammonia is a colourless gas with a distinct pungent smell. C Nitrogen from the petrol reacts with oxygen at the high temperatures in the engine. The autoxidation of formaldehyde through initiation by triplet oxygen is studied via two initial steps.
Nitrogen is the chemical element with the symbol N and atomic number 7. The H 2 H 2 O ratio of 22 could have been used also. In that case the ratio from the problem would have been 300 over x since you were now using the water data and not the oxygen data.
Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table often called the pnictogens. Ethane - Thermophysical Properties - Chemical Physical and Thermal Properties of Ethane - C 2 H 6. The reaction energy profiles show that the reactions are thermodynamically and kinetically demanding.
Where g acceleration due to gravity units typically ms 2 and value on Earth usually given as 980665 ms 2 or 3217405 fts 2 Tabulated values of ammonia density at given temperature and atmospheric pressure SI and Imperial units are given below the figures. D Nitrogen reacts with petrol. Notice that the above solution used the answer from example 5.
The solution below uses the information given in the original problem. A Nitrogen and oxygen from the air react together at the high temperatures in the engine. How are these oxides formed.
A carbon dioxide and oxygen B carbon dioxide only C nitrogen and oxygen D nitrogen only 31 The gases coming from a cars engine contain oxides of nitrogen. The ammonia that produced is removed by refrigerating it until it. Two nitrogen atoms combine with a triple bond to compose a nitrogen molecule.
The fixation of nitrogen is an effective approach to achieve the nitrogen cycle in the ecosystem. 2NI₃s 3I₂g N₂g. How are these oxides of nitrogen formed.
X 300 mol of H 2 was consumed. For example nitrogen triiodide NI₃ decomposes explosively into purple iodine vapour and nitrogen gas when touched by a feather. Industrially the conversion of nitrogen to ammonia is accomplished by the Haber synthesis Fritz Haber 1905 Nobel Prize in Chemistry 1918 in which nitrogen gas reacts with hydrogen gas at temperatures of around 400C at 200 to 300 atmospheres of pressure using a catalyst of fused MgO Al 2 O 3 and SiO 2.
B Nitrogen reacts with carbon monoxide. A Nitrogen reacts with carbon dioxide.
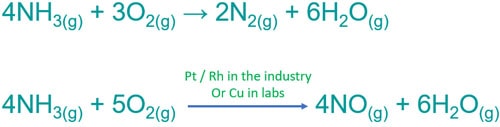
Ammonia Oxygen Reaction Nh3 O2 Balanced Equation
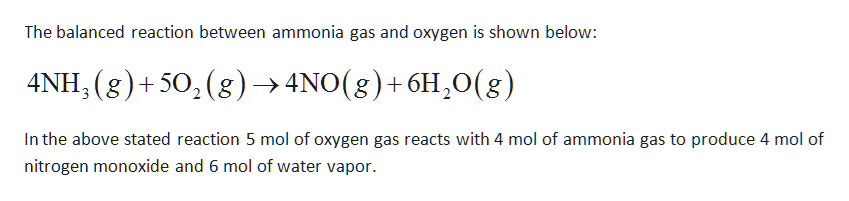
Answered Gaseous Ammonia Chemically Reacts With Bartleby

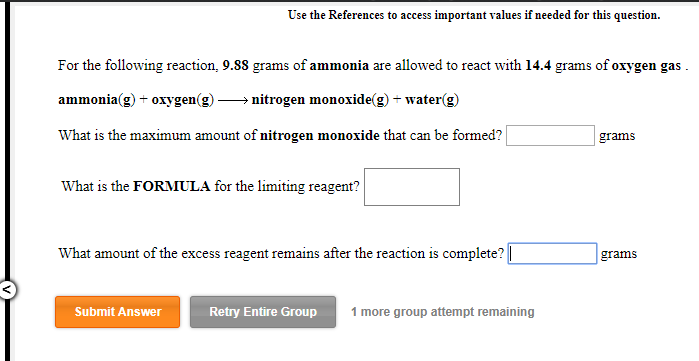
Solved Use The References To Access Important Values If Chegg Com

No comments for "Ammonia G Oxygen G Nitrogen Monoxide G Water G"
Post a Comment